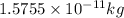Answer:
For 3: The total mass change of the reaction is
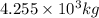
For 4: The mass defect is
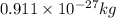 and energy equivalent to this mass is
and energy equivalent to this mass is
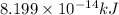
For 5: The equivalent mass of the reaction is
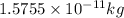
Step-by-step explanation:
To calculate the mass change of the reaction for given energy released, we use Einstein's equation:

E = Energy released =
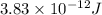
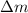 = mass change = ?
= mass change = ?
c = speed of light =
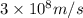
Putting values in above equation, we get:
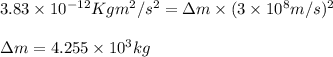
Hence, the total mass change of the reaction is
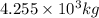
For the given isotopic representation:
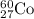
Atomic number = Number of protons = 27
Mass number = 60
Number of neutrons = Mass number - Atomic number = 60 - 27 = 33
To calculate the mass defect of the nucleus, we use the equation:
![\Delta m=[(n_p* m_p)+(n_n* m_n)+]-M](https://img.qammunity.org/2019/formulas/chemistry/high-school/p5gkhxeu8z85pbxbqj1zeyvo4icqd82gyf.png)
where,
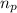 = number of protons = 27
= number of protons = 27
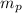 = mass of one proton = 1.00728 amu
= mass of one proton = 1.00728 amu
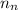 = number of neutrons = 33
= number of neutrons = 33
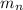 = mass of one neutron = 1.00867 amu
= mass of one neutron = 1.00867 amu
M = Nuclear mass number = 59.9338 amu
Putting values in above equation, we get:
![\Delta m=[(27* 1.00728)+(33* 1.00867)]-[59.9338]\\\\\Delta m=0.54887amu](https://img.qammunity.org/2019/formulas/chemistry/high-school/q0luux1unawnhk96qb6erom6wsdjbrz5kl.png)
Converting the value of amu into kilograms, we use the conversion factor:
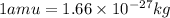
So,
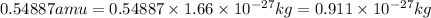
To calculate the equivalent energy, we use the equation:

E = Energy released = ?
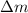 = mass change =
= mass change =
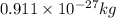
c = speed of light =
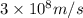
Putting values in above equation, we get:
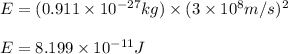
Converting this into kilojoules, we use the conversion factor:
1 kJ = 1000 J
So,
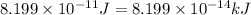
Hence, the mass defect is
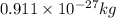 and energy equivalent to this mass is
and energy equivalent to this mass is
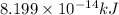
For the given chemical reaction:
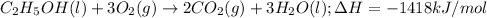
To calculate the equivalent mass of the reaction for given energy released, we use Einstein's equation:

E = Energy released =
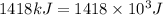
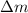 = mass change = ?
= mass change = ?
c = speed of light =
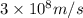
Putting values in above equation, we get:
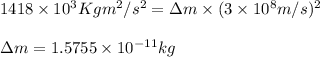
Hence, the equivalent mass of the reaction is