Answer:
Hydrogen bonding
Step-by-step explanation:
Hydrogen bonding is a type of dipole dipole interaction. It exist between H atom bonded to electronegative atom; O, N, F and other O, N, F of another molecule.
Some of the examples of compounds having hydrogen bonding are:
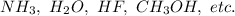
Hydrogen bonding exists because of development of dipole dipole interaction between H and electronegative atom.
As H is less electronegative as compared to O, N and F. So partial positive charge develop on H and partial negative chagre develop develop on the attached electronegative atom. Because of development of partial positive and partial negative charge, dipole dipole interaction occurs which leads to the development of hydrogen bonding.