In any adiabatic system we always say that exchange of heat in that system must be zero
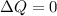
so from first law we have
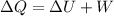
so we have
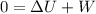
so here we can say that negative change in internal energy is equal to work done by the system.
so the correct answer must be
A. In an adiabatic process, no heat enters or leaves the system.