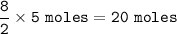Moles of Carbondioxide-CO₂ produced = 20 moles
Further explanation
The combustion of hydrocarbons with excess oxygen will produce carbon dioxide and water(CO₂+H₂O), whereas if there is not much oxygen, carbon monoxide and water(CO+H₂O) will be obtained.
The reaction coefficient in a chemical equation shows the mole ratio of the reacting compounds
Reaction (combustion of butane) :
2C₄H₁₀+13O₂⇒8CO₂+10H₂O
Butane reacts completely, then Butane is the limiting reactant and oxygen as the excess reactant, so the moles of Carbon dioxide are based on the butane moles as the limiting reactant.
moles of butane - C₄H₁₀ = 5 moles
From the reaction, the mol ratio of C₄H₁₀ and CO₂ : 2 : 8, so mol CO₂ :