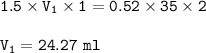The volume of HCl = 24.27 ml
Further explanation
Reaction
2HCl + Ba(OH)₂ ⇒ BaCl₂ +2 H₂O
mol of Ba(OH)₂ = V x M
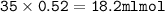
From the equation, the mol ratio of HCl : Ba(OH)₂ = 2 : 1, so mol HCl :
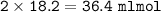
So the volume of HCl :
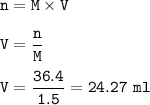
Or you can use the titration formula :
M₁V₁n₁=M₂V₂n₂(n HCl=1, n Ba(OH)₂=2) :