The temperature : 332.75 K
Further explanation
Conditions at T 0 ° C and P 1 atm are stated by STP (Standard Temperature and Pressure). At STP, Vm is 22.4 liters / mol.
volume of gas = 790 cm³ = 0.79 L
mol of gas at STP :
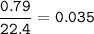
Use ideal gas :
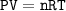
P=726 mmHg=0.955 atm
V= 1000 cm³ = 1 L
