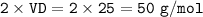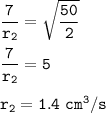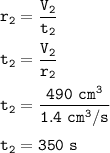The time taken by gas x : 350 s
Further explanation
Graham's law: the rate of effusion of a gas is inversely proportional to the square root of its molar masses or
the effusion rates of two gases = the square root of the inverse of their molar masses:
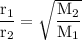
Molar mass = 2 x vapour density(VD)
r₁=rate Hydrogen = 280 cm³ / 40 s = 7 cm³/s
M₁=molar mass Hydrogen=2 g/mol
M₂=molar mass gas x=