Answer:
0.94 moles
Step-by-step explanation:
To find the number of moles in a substance given it's number of entities we use the formula
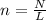
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
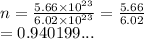
We have the final answer as
0.94 moles
Hope this helps you