It takes 333.3 s for Oxygen to diffuses
Further explanation
Graham's law: the rate of effusion of a gas is inversely proportional to the square root of its molar masses or
the effusion rates of two gases = the square root of the inverse of their molar masses:
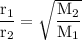
or
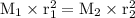
r₁ H₂ = 300 cm³/50 s=6 cm³/s
M₁ H₂ = 2 g/mol
M₂ O₂ = 32 g/mol

the diffusion time of Oxygen :
