Answer:
44.59°c
Step-by-step explanation:
Given data :
Total pressure = 105 kpa
complete combustion
A) Determine air-fuel ratio
A-F =
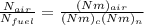
N = number of mole
m = molar mass
A-F =
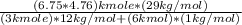 = 22.2 kg air/fuel
= 22.2 kg air/fuel
hence the ratio of Fuel-air = 1 : 22.2
B) Determine the temperature at which water vapor in the products start condensing
First we determine the partial pressure of water vapor before using the steam table to determine the corresponding saturation temp
partial pressure of water vapor
Pv =
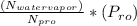
N watervapor ( number of mole of water vapor ) = 3
N pro ( total number of mole of product = 3 + 3 + 2.25 + 25.28 = 33.53 kmol
Pro = 105
hence Pv = ( 3/33.53 ) * 105 = 9.39kPa
from the steam pressure table the corresponding saturation temperature to 9.39kPa = 44.59° c
Temperature at which condensing will start = 44.59°c
An equation showing the products of propylene with their mole numbers is attached below