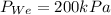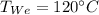Answer:
120°C
Step-by-step explanation:
Step one:
given data
T_{wi} = 20^{\circ}C
T_{Ai}=1000K
T_{Ae}= 400kPa
P_{Wi}=200kPa
P_{Ai}=125kPa
P_{We}=200kPa
P_{Ae}=100kPa
m_A=2kg/s
m_W=0.5kg/s
We know that the energy equation is
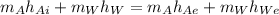
making
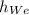 the subject of formula we have
the subject of formula we have
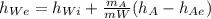
from the saturated water table B.1.1 , corresponding to
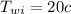
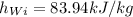
from the ideal gas properties of air table B.7.1 , corresponding to T=1000K
the enthalpy is:
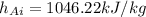
from the ideal gas properties of air table B.7.1 corresponding to T=400K
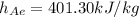
Step two:
substituting into the equation we have
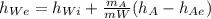
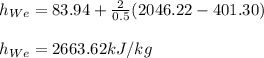
from saturated water table B.1.2 at
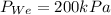 we can obtain the specific enthalpy:
we can obtain the specific enthalpy:
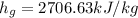
we can see that
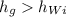 , hence there are two phases
, hence there are two phases
from saturated water table B.1.2 at