Answer:
Atomic orbitals describe where an electron is likely to be found in an atom.
Every atomic orbital is associated with three quantum numbers, n, l, and ml, which describe the energy, angular momentum of the orbital's electron(s), and orientation of the orbital.
The "sub shells" are the orientations and shapes for orbitals. Essentially, they are a subdivision of electron shells, separated by electron orbitals.
Same
 and
and
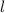 → same sub-shell
→ same sub-shell
Same
 ,
,
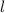 , and
, and
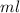 → same orbital
→ same orbital
All electrons that share the same orbital shape within the same shell/energy level, are in the same sub-shell. When electrons share the same energy level, shape, and orientation, they are in the same orbital.
This is a very brief recap of subshells and orbitals.