Answer:
Sigma Bonds = 14
Pi bonds = 0
Step-by-step explanation:
Butanol:
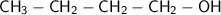
There is no pi bond in this compound (since there is no double or triply bond)
So,
Pi bond = 0
Now, Coming towards sigma bonds
Sigma bonds = 3 (First carbon with hydrogen) + 2 (Second C with H) + 2 (Third C with H) + 2 (Fourth C with H) + 1 (Bond of O and H) + 4 (Bonds between C and C and C and O)
Sigma Bonds = 14
![\rule[225]{225}{2}](https://img.qammunity.org/2021/formulas/chemistry/middle-school/ri1osubq0ul0fyszs3s2hvchlv85n22xxb.png)
Hope this helped!
~AnonymousHelper1807