Given :
The atomic weight of copper is 63.5 gm.
To Find :
The total number of gram atoms and number of atoms present in 100 gram of copper.
Solution :
We know, 1 gram atom is equal to mass of one mole of copper i.e. 63.5 gm.
So, number of gram atom in 100 gram of copper is :
n = 100/63.5
n = 1.57 gram atom
Now, number of atoms on gram atom is :
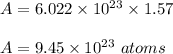
Therefore, total number of gram atoms and number of atoms present in 100 gram of copper is 1.57 gram atom and
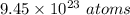 .
.
Hence, this is the required solution.