Answer:
Percentage yield =58.7 %
Step-by-step explanation:
moles of calcium carbonate as a reactant =
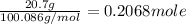
as per molar equation , 0.2068 moles of calcium carbonate produces 0.2068 moles of CaO .
amount of CaO = 0.2068 mole x (40.078+15.999) g/moles = 11.598 gram
percentage yield = actual yield / theoritical yield = 6.81/ 11.598 = 0.587 or 58.7 %