Answer:
0.0035M
Step-by-step explanation:
Given parameters
Volume of HCl = 100mL = 0.1L
Volume of LiOH = 46.9mL = 0.0469L
Molarity of LiOH = 0.75M
Unknown:
Concentration of hydrochloric acid = ?
Solution:
The reaction equation is given as;
HCl + LiOH → LiCl + H₂O
Let us find the number of moles of the given specie which is LiOH;
Number of moles = molarity x volume;
Number of moles of LiOH = 0.75 x0.0469 = 0.035moles
From the balanced reaction equation;
1 mole of LiOH combines with 1 mole of HCl
0.035mole of LiOH will require 0.035mole of HCl
Concentration of HCl =
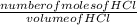
Concentration of HCl =
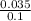 = 0.0035M
= 0.0035M