Answer:
Multiply 1.25 by 0.04 and divide the result obtained by 1,000
Step-by-step explanation:
Given parameter:
Density of the gas:
1.25 g/L
Now the problem here is to convert to ounce/milliliter;
1gram = 0.04ounce
1 liter = 1000mL
So;
1.25g/ L x
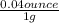 x
x
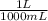 = 5 x 10⁻⁵ounce/mL
= 5 x 10⁻⁵ounce/mL