Answer:
11.541 mol/min
Step-by-step explanation:
temperature = 35°C
Total pressure = 1.5 * 1.013 * 10^5 = 151.95 kPa
note : partial pressure of water in mixture = saturation pressure of water at T = 35°c )
from steam table it is = 5.6291 Kpa
calculate the mole fraction of H
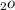 ( YH
( YH
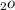 )
)
= 5.6291 / 151.95
= 0.03704
calculate the mole fraction of air ( Yair )
= 1 - mole fraction of water
= 1 - 0.03704 = 0.9629
Now to determine the molar flow rate of water vapor in the stream
lets assume N = Total molar flow rate
NH
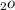 = molar flow rate of water
= molar flow rate of water
Nair = molar flow rate of air = 300 moles /min
note : Yair * n = Nair
therefore n = 300 / 0.9629 = 311.541 moles /min
Molar flowrate of water
= n - Nair
= 311.541 - 300 = 11.541 mol/min