Answer:
pH = 12.11
Step-by-step explanation:
Hello!
In this case, since the chemical reaction between perchloric acid and lithium hydroxide is:

Whereas the mole ratio between the acid and base is 1:1, it means they react in the same proportion. In such a way, given the volume and concentration of acid, we compute the moles:
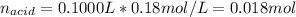
Now, the moles of base that reacted:
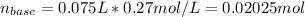
Thus, since there is an excess of lithium hydroxide of:
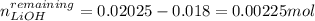
We compute the new concentration considering the total final volume of 175.0 mL:
![[LiOH]=(0.00225mol)/(0.1750L)=0.013M](https://img.qammunity.org/2021/formulas/chemistry/college/8qz1r7zehurwfkg0ub7qw1yu7l5i0etu5l.png)
Now, since there is a strong base remaining in solution, we compute its pOH first:
![pOH=-log([OH^-])=-log(0.013M)=1.89](https://img.qammunity.org/2021/formulas/chemistry/college/r7empw8kilwxtowbygp5hog3ghn11lftxb.png)
Because LiOH is fully ionized to Li⁺ and OH⁻ ions. Therefore the pH is:
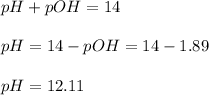
Best regards!