Answer:

Step-by-step explanation:
Density can be found by dividing the mass by the volume.
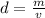
The mass of the rock is 555 grams and the volume is 101.1 cubic centimeters.
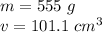
Substitute the values into the formula.
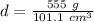
Divide.
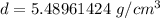
Let's round to the nearest hundredth to make the answer more concise.
The 9 in the thousandth place tells us to round up. In the tenth place, the 8 will become a 9.
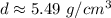
The density of the rock is about 5.49 grams per cubic centimeter.