Answer:

Step-by-step explanation:
✑ First, Let's explore about ATOMIC MASS :
↱ An atom consists of electrons , protons and neutrons. The mass of electron is extremely small and it is taken as a negligible mass compared to the mass of a proton or neutron. Thus , The atomic mass is defined as the sum of the number of protons and neutrons present in the nucleus of an atom of that element. If is also called atomic weight. It is denoted by A. The nucleus determines the mass of an atom, while the number of electrons determines the size of the atom of the element.
Mathematically,
Atomic mass = Number of protons + Number of neutrons
i.e
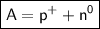
———————————————————————
✎ Now , Let's find the atomic mass of atom having 6 protons and 8 neutrons :
☞ Atomic mass = No. of protons + No. of neutrons
➵ Atomic mass = 6 + 8
➵ Atomic mass =

And we're done !
Hope I helped!
Have a wonderful day! ツ
~TheAnimeGirl ♡
▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁▁