Answer: hydrogen is the limiting reactant.
Step-by-step explanation:
We have the equation
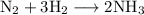 .
.
This means that for every mole of nitrogen consumed, 3 moles of hydrogen are consumed.
- Considering the nitrogen, the reaction can occur 0.50 times.
- Considering the hydrogen, the reaction can occur 1.8/3 = 0.6 times.
Therefore, hydrogen is the limiting reactant.