Answer:
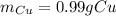
Step-by-step explanation:
Hello!
In this case, considering the given chemical reaction and the mass of the magnesium strip, following the indications of the atomic weight ratio (2.61 g Cu/1 g Mg), and keeping in mind the 1:1 mole ratio one could compute the produced mass of copper as shown below:
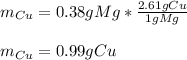
Best regards!