Answer:
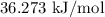
Step-by-step explanation:
m = Mass of LiCl = 2.5 g
M = Molar mass of LiCl = 42.394 g/mol
c = Specific heat of water =
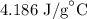
 = Change in temperature =
= Change in temperature =
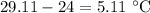
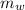 = Mass of water =
= Mass of water =
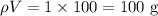
Number of moles
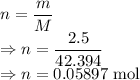
Heat is given by
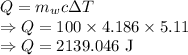
Enthalpy is given by
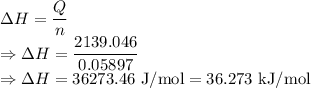
The enthalpy for the dissolution is
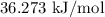 .
.