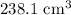Answer:
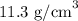
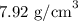
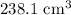
Step-by-step explanation:
8) Volume of lead
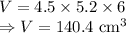
m = Mass of block = 1587 g
Density is given by
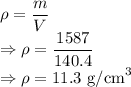
Density of lead is
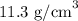
9) Volume of lead = Volume of water displaced = (49.1-45.5) = 3.6 mL =
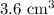
m = Mass of iron = 28.5 g
Density is given by
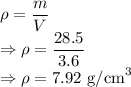
The density of iron is
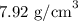
10) m = Mass of silver = 2500 g
 = Density of silver =
= Density of silver =
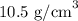
Volume is given by
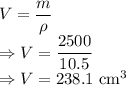
The volume of silver is