Step-by-step explanation:
For the first part,
Reaction equation:
N₂ + 3H₂ → 2NH₃
Given:
Number of moles of NH₃ = 6 moles
Unknown:
Number of moles of N₂ = ?
Solution:
N₂ + 3H₂ → 2NH₃;
From the reaction above, we solve from the known specie to the unknown. Ensure that the equation is balanced;
2 moles of NH₃ is produced from 1 mole of N₂
6 moles of NH₃ will be produced from
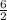 mole of N₂
mole of N₂
= 3moles of N₂
The number of moles of N₂ is 3 moles
ii.
Given parameters:
Number of moles of sulfur = 2.4moles
Molar mass of sulfur = 32.07g/mol
Unknown:
Mass of sulfur = ?
Solution:
The number of moles of any substance can be found using the expression below;
Number of moles =

Mass of sulfur = number of moles of sulfur x molar mass
Insert the parameters and solve;
Mass of sulfur = 2.4 x 32.07 = 76.97g