Answer:
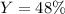
Step-by-step explanation:
Hello!
In this case, since the described chemical reaction is:
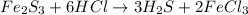
We can see the 6:2 mole ratio between hydrogen chloride (molar mass = 36.46 g/mol) and hydrogen sulfide (molar mass = 34.09 g/mol) and 1:3 between iron (III) sulfide (molar mass = 207.91 g/mol) and hydrogen sulfide. In such a way, we can compute the yielded moles of hydrogen sulfide by each reactant in order to identify the limiting one:
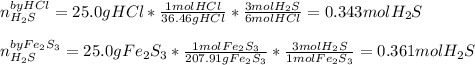
It means that the HCl is the liming reactant as it produces less moles of hydrogen chloride than the iron (III) sulfide. Therefore, the theoretical yield in grams is:
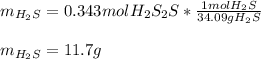
Thus, the percent yield is:
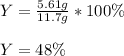
Best regards!