Answer:
Yes it is possible.
Step-by-step explanation:
This problem is about to possibility to have alloy of iron-carbon for which mass fraction of ferrite,
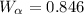 and proeutectoid cementite,
and proeutectoid cementite,
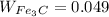
An alloy formation is possible when the composition values of the two alloy are equal.
Now writing the expression for the mass fraction of total ferrite, we have
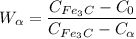
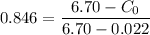
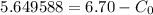
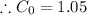 wt. % of C
wt. % of C
Now write the expression for the mass fraction of the proeutectoid cementite :
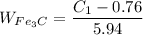
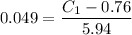
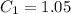 % wt. C
% wt. C
Since,
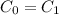 , it is possible to have an alloy of iron - carbon.
, it is possible to have an alloy of iron - carbon.