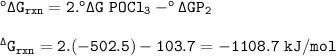°ΔG rxn = -1108.7
Further explanation
Maybe the question is like this
the value of ΔG° at 25 °C for the formation of POCl₃
Reaction
P₂(g)+O₂(g)+3Cl₂(g)⇒2POCl₃(g)
Formula
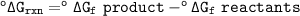
You can find table to find ΔG° at 25 °C(298 K)
°ΔG POCl₃ = -502.5 kJ/mol
°ΔG O₂ and °ΔG Cl₂ = 0
°ΔG P₂ = 103.7 kJ/mol