Step-by-step explanation:
Given parameters:
Wavelength of photon = 1.95 x 10⁻¹¹m
Unknown:
Frequency of the wave = ?
Energy of the photon = ?
Solution:
The frequency and wavelength of a wave are related by the expression;
C = Fλ
C = speed of light = 3 x 10⁸m/s
F = frequency
λ = wavelength
Insert the parameters and solve;
3 x 10⁸ = F x 1.95 x 10⁻¹¹
F =
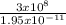 = 1.54 x 10¹⁹Hz
= 1.54 x 10¹⁹Hz
Energy of the photon is related to frequency using the expression below;
E = hF
h is the Planck's constant = 6.634 x 10⁻³⁴m²kg/s
Insert the parameters and solve;
E = 6.634 x 10⁻³⁴ x 1.54 x 10¹⁹ = 1.02 x 10⁻¹⁴J