Answer:
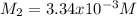
Step-by-step explanation:
Hello!
In this case, since a dilution process implies that the moles of the solute remain the same before and after the addition of diluting water, we can write:

Thus, since we know the volume and concentration of the initial sample, we compute the resulting concentration as shown below:
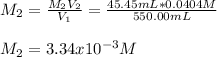
Best regards!