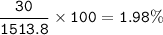The concentration, mass/volume percent (m/v) : 1.98%
Further explanation
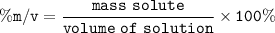
mass solute = mass of NaCl = 30 g
volume of solution = volume of water + volume of NaCl
volume of water = 1.5 L = 1500 ml
volume of NaCl :(density = 2.16 g/ml)
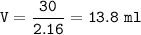
volume of solution :
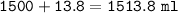
the concentration (%m/v) :