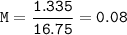Molarity of the solution = 0.08 M
Further explanation
Molarity is a way to express the concentration of the solution
Molarity shows the number of moles of solute in every 1 liter of solute or mmol in each ml of solution
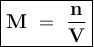
Where
M = Molarity
n = Number of moles of solute
V = Volume of solution
0.5 lb=226,796 g
MW silver nitrat - AgNO₃ = 169,87 g/mol
mol AgNO₃ :
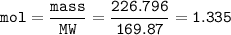
Molarity :