Answer:
A. The balloons will increase to twice their original volume.
Step-by-step explanation:
Boyle's law states that the pressure exerted on a gas is inversely proportional to the volume occupied by the gas at constant temperature. That is:
P ∝ 1/V
P = k/V
PV = k (constant)
P = pressure, V = volume.

Let the initial pressure of the balloon be P, i.e.
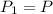 , initial volume be V, i.e.
, initial volume be V, i.e.
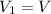 . The pressure is then halved, i.e.
. The pressure is then halved, i.e.

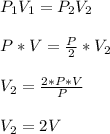
Therefore the balloon volume will increase to twice their original volume.