Answer:
A. fluorine, 1.79 moles
Step-by-step explanation:
Given parameters:
Mass of carbon = 87.7g
Mass of fluorine gas = 136g
Unknown:
The limiting reactant and the maximum amount of moles of carbon tetrafluoride that can be produced = ?
Solution:
Equation of the reaction:
C + 2F₂ → CF₄
let us find the number of the moles the given species;
Number of moles =

C; molar mass = 12;
Number of moles =
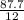 = 7.31moles
= 7.31moles
F; molar mass = 2(19) = 38g/mol
Number of moles =
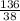 = 3.58moles
= 3.58moles
So;
From the give reaction:
1 mole of C requires 2 moles of F₂
7.31 moles of C will then require 2 x 7.31 moles of F₂ = 14.62moles
But we have 3.58 moles of the F₂;
Therefore, the reactant in short supply is F₂ and it is the limiting reactant;
So;
2 moles of F₂ will produce mole of CF₄
3.58 moles of F₂ will then produce
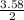 = 1.79moles of CF₄
= 1.79moles of CF₄