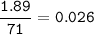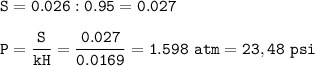The pressure : 23.48 psi
Further eplanation
Henry's Law stated that the solubility of a gas is proportional to its partial pressure
Can be formulated
S = kH. P.
S = gas solubility, mol / L
kH = Henry constant, mol / L.atm
P = partial gas pressure
MW Cl₂ = 71 g/mol
mol of 1.23 g Cl₂ :
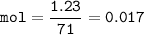
Solubility O₂ : 0.017 mol/0.95 L =0.01789 mol/L
Henry's constant for O₂ :
P = 15.6 psi= 1.061517 atm