Answer:
The new element will be thorium-226 (²²⁶Th).
Step-by-step explanation:
The beta decay is given by:
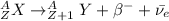
Where:
A: is the mass number
Z: is the number of protons
β⁻: is a beta particle = electron
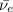 : is an antineutrino
: is an antineutrino
The neutral atom has 88 electrons, so:
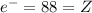
Hence the element is radium (Ra), it has A = 226.
If Ra undergoes 2 rounds of beta minus decay, we have:
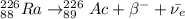
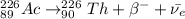
Therefore, if a neutral atom with 88 electrons undergoes 2 rounds of beta minus decay the new element will be thorium-226 (²²⁶Th).
I hope it helps you!