Answer:
The efficiency of this fuel cell is 80.69 percent.
Step-by-step explanation:
From Physics we define the efficiency of the automotive fuel cell (
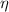 ), dimensionless, as:
), dimensionless, as:
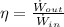 (Eq. 1)
(Eq. 1)
Where:
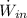 - Maximum power possible from hydrogen flow, measured in kilowatts.
- Maximum power possible from hydrogen flow, measured in kilowatts.
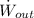 - Output power of the automotive fuel cell, measured in kilowatts.
- Output power of the automotive fuel cell, measured in kilowatts.
The maximum power possible from hydrogen flow is:
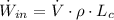 (Eq. 2)
(Eq. 2)
Where:
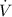 - Volume flow rate, measured in cubic meters per second.
- Volume flow rate, measured in cubic meters per second.
 - Density of hydrogen, measured in kilograms per cubic meter.
- Density of hydrogen, measured in kilograms per cubic meter.
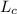 - Heating value of hydrogen, measured in kilojoules per kilogram.
- Heating value of hydrogen, measured in kilojoules per kilogram.
If we know that
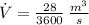 ,
,
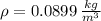 ,
,
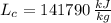 and
and
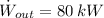 , then the efficiency of this fuel cell is:
, then the efficiency of this fuel cell is:
(Eq. 1)
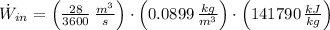
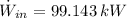
(Eq. 2)
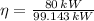
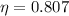
The efficiency of this fuel cell is 80.69 percent.