Answer:
The necessary number of electron charge carriers required is:
8.1019 × 10¹⁹ electrons/m³
Step-by-step explanation:
The necessary number of charge carriers required can be determined from the resistivity. Given that, the phosphorus make an n-type of silicon semiconductor;
Resistivity
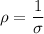
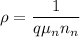
where;
The number of electron on the charge carriers
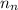 is unknown??
is unknown??
The charge of the electron q =
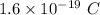
The electron mobility
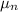 = 0.135 m²/V.s
= 0.135 m²/V.s
The electrical conductivity
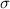 = 1.75 (Ωm)⁻¹
= 1.75 (Ωm)⁻¹
Making
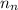 the subject from the above equation:
the subject from the above equation:
Then;
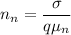
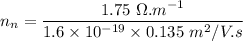
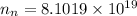 electrons/m³
electrons/m³
Thus; the necessary number of electron charge carriers required is:
8.1019 × 10¹⁹ electrons/m³