Answer: 558.5545 g
Step-by-step explanation:
The formula for a sulphate ion is
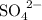 , so the formula for copper sulphate is
, so the formula for copper sulphate is
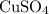 .
.
- The atomic mass of copper is 63.546 g/mol.
- The atomic mass of sulfur is 32.065 g/mol.
- The atomic mass of oxygen is 15.994 g/mol.
This means the formula mass of copper sulphate is:
- 63.546+32.065+4(15.994)=159.587 g/mol.
So, in 3.5 moles, the mass is (159.587)(3.5)=558.5545 g