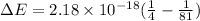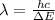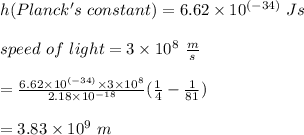Answer:
The answer is "
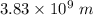 "
"
Step-by-step explanation:
Z=2, so the equation is
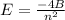
Calculate the value for E when:
n=2 and n=9
The energy is the difference in transformation, name the energy delta E Deduct these two energies
In this transition, the wavelength of the photon emitted is: