Answer:
38.7%
41.3%
20%
Step-by-step explanation:
The percentage composition helps to know the what percent of the total mass of a compound is made up of each of the constituent elements or groups.
To solve this problem:
- find the formula mass by adding the atomic masses of the atoms that makes up the compound.
- place the mass contribution of the element or group to the formula mas and multiply by 100;
Compound:
Ca₃(PO₄)₂
Formula mass = 3(40) + 2[31 + 4(16)]
= 120 + 2(95)
= 120 + 190
= 310
%C =
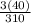 x 100 = 38.7%
x 100 = 38.7%
%P =
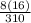 x 100 = 41.3%
x 100 = 41.3%
%O =
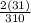 x 200 = 20%
x 200 = 20%