Answer:
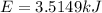
Step-by-step explanation:
Hello.
In this case, for such combustion of hexane which release heat, we can compute that amount by taking into account that the reaction releases 4163.0 kJ of energy every 1 mole of hexane, it means that for 72.78 g, which in moles is:
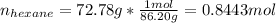
Thus the energy released when 0.8443 moles of hexane are released turn out:
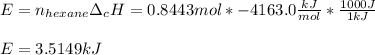
Best regards!