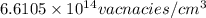Answer:
The concentration of vacancies in fcc silver is
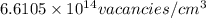
Step-by-step explanation:
Given is - silver (fcc lattice)
Temperature = 250°C = 523K
Activation energy for vacancy creation ,
 =79.5 kJ/mol
=79.5 kJ/mol
Silver has a fcc crystal lattice structure with basis
Lattice parameter = 0.49nm =
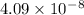 m
m
Now , let us calculate number of silver atoms"(lattice points)per
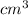
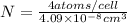
=
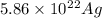 atoms/
atoms/
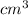
Concentration of vacancies is given by the formula -
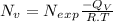
here,
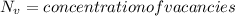
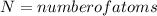
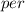
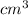
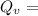 Activation energy for vacancy creation
Activation energy for vacancy creation
T = Temperature
R = constant =
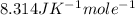
Now , putting the given values in the formula -
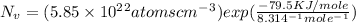
=
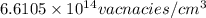
Hence , the answer is