Answer:
Both empirical and molecular formulas are NH₃.
Step-by-step explanation:
Hello.
In this case, since we know the percent compositions of both nitrogen and hydrogen, in order find out the molecular formula and the empirical formula of that compound, we can first assume we have 82.27 g of nitrogen and 17.76 g of hydrogen as a first approximation to compute the present moles:
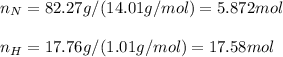
Next, we compute the subscripts in the empirical formula first, by diving the moles of each element by the moles of the element with fewest moles:
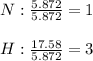
It means that the empirical formula is NH₃ and its molar mass is about 17.04 g/mol which is about the same the reported molar mass slightly more than 17 g, it means that the molecular formula is also NH₃.
Best regards!