Answer:
Formula for the strongest conjugate base is
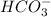 , that is hydrogen carbonate . As we know that a string acid has the weakest conjugate bases, then we come to know that F- is the weakest conjugate base when we compare the relative acidic strength of Hf, H2CO3,H2S.
, that is hydrogen carbonate . As we know that a string acid has the weakest conjugate bases, then we come to know that F- is the weakest conjugate base when we compare the relative acidic strength of Hf, H2CO3,H2S.
Therefore,
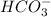 has a powerful conjugate base,
has a powerful conjugate base,
Step-by-step explanation:
HYDROGEN CARBONATE -: In the deprotonation of carbonic acid and a polyatomic anion, hydrogen carbonate, also known as bicarbonate and amphoteric in nature, is an intermediate form. In the physiological pH buffering system, bicarbonate serves a crucial biochemical role.