Answer:
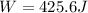
Step-by-step explanation:
Hello.
In this case, the work for such expansion is computed via:
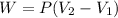
Since it is an expansion, we infer that the final volume is greater than the initial one thereby the gas makes work so it is positive. It means that the work is:
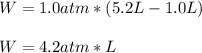
Which in J it is:
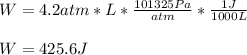
Best regards!