Answer:
The concentration of the dilute solution is 0.7323 M
Step-by-step explanation:
A solution is to prepare a solution from having the solute and the solvent initially separated, that is, it is the homogeneous mixture of two or more substances.
On the other hand, dilution is the procedure that is followed to prepare a less concentrated solution from a more concentrated one, and consists of adding more solvent to the solution.
In a dilution, the amount of solute does not change, but the volume of the solvent varies, that is, as more solvent is added, the concentration of the solute decreases because the volume (and weight) of the solution increases.
In a dilution it is fulfilled:
Ci*Vi = Cf*Vf
where:
- Ci: initial concentration
- Vi: initial volume
- Cf: final concentration
- Vf: final volume
In this case:
- Ci: 5.95 M
- Vi: 100 mL= 0.1 L (being 1,000 mL= 1 L)
- Cf: ?
- Vf: 812.5 mL= 0.8125 L
Replacing:
5.95 M* 0.1 L= Cf* 0.8125 L
Solving:
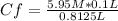
Cf= 0.7323 M
The concentration of the dilute solution is 0.7323 M