Complete Question
Calculate the wavelength, in meters, associated with a 56g golf ball moving at 20. m/s (about 45 mph).
Answer:
The value is

Step-by-step explanation:
From the question we are told that
The speed of the golf ball is
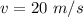
The mass is
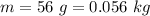
Generally from Broglie's equation we have that
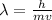
Here h is the Planck constant with value
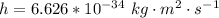
Here
 is the wavelength associated with the golf ball
is the wavelength associated with the golf ball
So
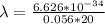
=>
