Sodium would have the highest average kinetic energy
Further explanation
Kinetic Molecular Theory (KMT) states that a gas consists of molecules that move at a constant and random speed. The collisions between molecules are perfectly elastic so that no energy is wasted.
The molecules move in straight lines until they collide
Energy because this motion is expressed as Kinetic energy (KE) which can be formulated as:
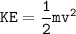
Average velocities of gases can be expressed as root-mean-square averages. (V rms)
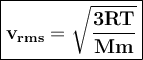
R = gas constant, T = temperature, Mm = molar mass of the gas particles
So
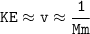
The substance would have the highest average kinetic energy = the lowest molar mass
Molar mass :
- Sodium : 22,989769 u
- Mercury : 200,59 u
- Neon : 200,59 u
- Magnesium : 24,305 u
From the data above, it shows that Sodium has the smallest molar mass so that it has the largest kinetic energy